ISO 22000:2018 Revisions Published, Sparks Explanation
No doubt that you know the International Organization for Standardization (ISO) has revised its Food Safety Management System – ISO 22000. What you may not know is the extent of the changes and how they impact your food facility. Regardless of size or sector, this release is applicable to all organizations in the food industry.
“Structuring documentation is now different, there’s a bigger responsibility for facilities to include stakeholders in various roles across the company, not just line workers or supervisors that oversee the food production.” said Alma Delia Hernández, Certification Manager at AIBI-CS. “In ISO 22000, definitions such as PPOs and critical control points were messy and lacked clarity. There were so many interpretations of these definitions, that it was easy to misunderstand.”
ISO 22000 has remained largely unchanged since 2005, despite the global evolution of food safety standards in recent years. “The complexity of publishing these changes was ultimately why ISO waited. ISO is now delivering unified and scalable standards, which will meet the needs to improve safety and sustainability in food production.” said Delia Hernández. The deadline for compliance is June 18, 2021, giving facilities three years to migrate to ISO 22000:2018.
Here are the main changes:
- The high-level structure (HLS): Revised from 8 to 10 regrouped clauses, it allows integration with other standards of management systems that keep the HLS, such as ISO 9001:2015, ISO 14001:2015, and ISO 45001:2018. Safety can be linked in a single system of quality, environmental, and occupational health.
- The meaning of some verbal forms: Terms like “shall”, “should”, “may” and “can” are clarified.
- “shall” indicates a requirement
- “should” indicates a recommendation
- “may” indicates a permission
- “can” indicates a possibility or a capability
- The terms and definitions: Organized alphabetically and there are more definitions than the previous version.
- The risk approach: Risk is a vital concept for food businesses and the standard will distinguish between risk at the operational level (through the Hazard Analysis Critical Control Point (HACCP) approach) and risk at the strategic level of the management system (business risk) with its ability to embrace opportunities in order to reach a business’s specific goals.
- The PDCA cycle: The standard will clarify the distinction between two Plan-Do-Check-Act (PDCA) cycles. In the new version, the different elements of the management system are organized according with the PDCA cycle; while the second, embedded within it, addresses the operations described in Clause 8, which simultaneously covers the principles of HACCP defined by the Codex Alimentarius.
The PDCA cycle can be described briefly as follows:
Plan: establish the objectives of the system and its processes, provide the resources needed to deliver the results, and identify and address risks and opportunities
Do: implement what was planned
Check: monitor and (where relevant) measure processes and the resulting products and services, analyze and evaluate information and data from monitoring, measuring, and verification activities, and report the results
Act: take actions to improve performance, as necessary
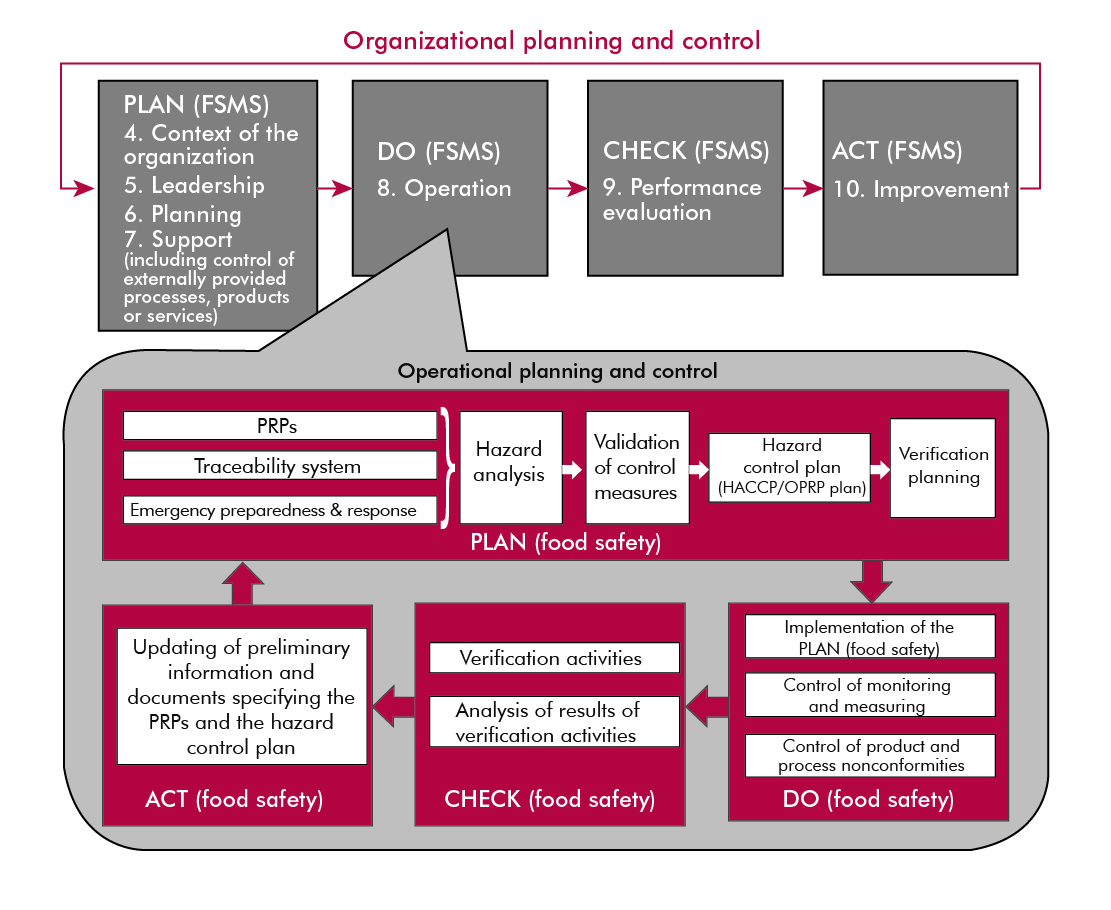
- The operation process: Differences between key terms are clarified, such as: Critical Control Points (CCPs), Operational Prerequisite Programs (OPRPs), and Prerequisite Programs (PRPs).
Critical Control Point (CCP) step in the process at which control measure(s) is (are) applied to prevent or reduce a significant food safety hazard to an acceptable level, and defined critical limit(s) and measurement enable the application of corrections
Operational Prerequisite Program (OPRP) control measure or combination of control measures applied to prevent or reduce a significant food safety hazard to an acceptable level, and where action criterion and measurement or observation enable effective control of the process and/or product.
Prerequisite Program (PRP) basic conditions and activities that are necessary within the organization and throughout the food chain to maintain food safety
*Note: The PRPs needed depend on the segment of the food chain in which the organization operates and the type of organization. Examples of equivalent terms are: good agricultural practice (GAP), good veterinary practice (GVP), good manufacturing practice (GMP), good hygiene practice (GHP), good production practice (GPP), good distribution practice (GDP) and good trading practice (GTP).
“Ensuring that our clients are prepared for the ISO compliance deadline is a key driver for AIBI-CS right now. We provide gap analysis, private training, and auditing services to help prepare food facilities for the future”, said Delia Hernández. “There are so many advantages for early adopters of ISO 22000:2018. We want to help clients make those changes now, not 2 months before the deadline.”
ISO 22000 is the backbone of the FSSC 22000 scheme, so this update requires a revision, which is expected in October 2018.
For additional information on AIBI-CS services, call us at 800-633-5137 or email here.
Thanks for sharing the valuable information about <a href="https://www.siscertifications.com/iso-22000-certification/">ISO 22000 Certification</a>


