Is the FDA Definition of 'Healthy' Changing? [Free Download]
In recent years, the Food and Drug Administration (FDA) proposed a new definition of “healthy” as it appears on food labels, in conjunction with the new nutrition facts panel. Since many Americans don't eat enough vegetables, fruits, or dairy, the FDA wanted to encourage increased consumption of these food groups while limiting the amount of added sugars, saturated fat, and sodium in Americans' diets.
The language of the proposed definition change is still under consideration, but it’s important to understand the suggested changes and the impact they might have on your food labeling.
The New FDA Definition of Healthy
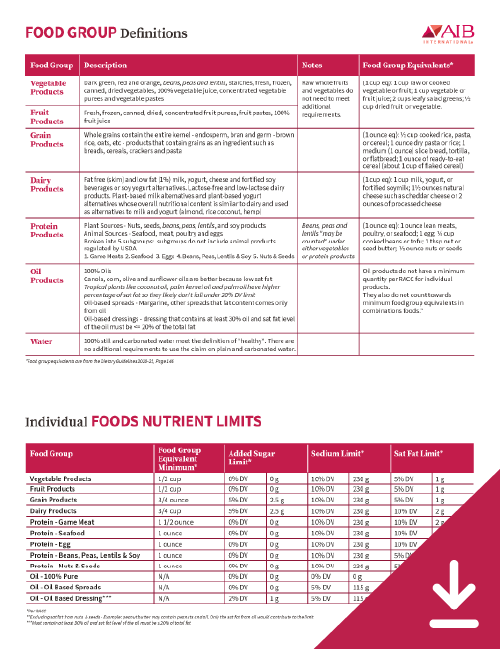
The FDA's new definition of “healthy” would be based on recommendations in the USDA's 2020 to 2025 Dietary Guidelines for Americans that state food must have:
- A minimum amount of at least one of the recommended food groups and maximum amounts of total fat, cholesterol, sodium, and saturated fat
- At least 10% of the daily value (DV) of key nutrients, including vitamin A, vitamin C, iron, calcium, protein, and fiber
The proposed rule includes exceptions for some products. Raw, whole fruits, and vegetables can use the claim “healthy” without meeting additional requirements. Pure still water and carbonated water can also use the claim without additional requirements if they are 100% water.
Food Product Categories
Under the proposed rule, food products are placed into the categories of individual foods or combination foods. Individual foods must meet a minimum amount of one food group and these minimum amounts are based on food group equivalents found in the Dietary Guidelines for 2020 to 2025. The nutrient limits for sodium and saturated fat all depend on the food group that the product contains.
Combination foods are defined as foods consisting of meaningful amounts of more than one food group. This category contains mixed products, main dish products, and meal products. In the proposed rule, products in this category contain more than one food group and must include minimum levels of food group equivalents based on the type of product. These foods have maximum allowed levels of added sugars, sodium, and saturated fat that are calculated using the nutrient limits of the food groups contained in the product.
A New “Healthy” Symbol
With so much consumer concern around food ingredients and contents, it’s become increasingly important to convey health information as symbols rather than just text. Already, careful consumers are used to looking for the symbols that represent “gluten-free,” “non-GMO,” and “organic,” to name a few. Regulators plan to create a similarly impactful and recognizable symbol for the new FDA definition of healthy.
Currently, the FDA is researching a symbol that would represent the “healthy” claim on food labels. They did not release additional information on what the symbol would be or the requirements for using it, but they did indicate they believe the updated definition of “healthy,” and a symbol, would help consumers easily identify healthier food choices.
Redefining 'Healthy' For a New Era
The new FDA definition of healthy has yet to attain full approval, but food companies still need to comply with current “healthy” requirements. AIB International has over a century of experience offering guidance and education on complying with FDA regulations — including food labeling requirements. Learn more in our Food Labeling Online course to keep your team up-to-date on the latest rules and mandates.

![Is the FDA Definition of 'Healthy' Changing? [Free Download]](https://blog.aibinternational.com/hubfs/Woman%20Reading%20food%20label%20in%20grocerty%20store%20blog%20header.png)