Environmental Monitoring Program: An Early Warning System for Microbiological Hazards

Although we enjoy one of the safest food supplies in the world, foodborne illness remains common in the United States where foodborne illness outbreaks affect millions and kill thousands of people. These outbreaks also undermine consumer confidence in affected products, result in large recalls, and diminish market demand.
A substantial number of foodborne illness outbreaks result from poor hygiene practices. Microorganisms can survive in food processing and handling environments. They are generally introduced into the food environment through raw materials, pests, air, water, and employees. Usually, the routine applications of good sanitation practices are able to control these microorganisms inside the food processing and handling environments. However, if contamination levels are high or sanitation procedures are inadequate, microorganisms may establish and can contaminate food products leading to foodborne illness outbreak.
Various pathogenic microorganisms such as E. coli, Listeria monocytogenes, and Salmonella spp. have well-established histories of being potential contaminants in food-handling environments. Hence, it is critical to monitor the hygienic environment in the food manufacturing facility for the production of high quality and safe food products. An environmental monitoring program (EMP) will assess the effectiveness of the overall hygienic practices in a facility and provide necessary information to prevent possible microbial contamination of food products. Keep in mind that EMP does not make food safe. Rather, it provides valuable data (source and concentration) on indicator organisms, spoilage organisms, and pathogens of concern in a timely manner.
To reduce the risk of microbial product contamination, one must have insight into implementing an effective EMP in a food facility and properly interpreting the data
to initiate appropriate corrective actions.
EMP Benefits
Some of the key benefits of EMP are that it:
- Measures the overall effectiveness of sanitary design, personnel practices, and operational methods.
- Provides information (source and concentration) about indicator organisms, spoilage organisms, and/or pathogens of concern in a timely manner, so that appropriate corrective actions can be initiated to prevent potential microbial outbreaks.
- Acts as an early warning system for microbiological hazards in both the production and post-production environment when well-developed and effectively implemented as an integral component of prerequisite programs.
- Helps to identify harborage niches and hot spots in a plant that may act as a source of contamination.
- Is a critical aspect of documenting the overall sanitary state of the facility.
- Validates the sanitation program and helps in determining the frequency required for cleaning and sanitation.
EMP is not designed to validate the effectiveness of cleaning and sanitizing methods, but is more focused on validating cleaning and sanitizing frequencies, and all the programs of the Good Manufacturing Practices (21 CFR).
An Effective EMP
The first task in the implementation of an EMP is to bring together individuals familiar with the operation to help identify potential areas of risk and concern in a facility. This group will be the EMP team and may include the quality manager, the plant or corporate microbiologist, line supervisors or operators, and sanitation supervisors or workers. If the facility does not have a food safety microbiology expert experienced in the development and implementation of an EMP, it is strongly recommended that the facility contact an experienced outside expert for guidance.
An EMP should be carefully designed after evaluating the facility and its products. Different food plants (e.g., plant vs. animal products, dry vs. wet processing facility) with various food products may require different EMPs. An EMP is specific to a facility and to the individual operations within a facility. A tailored environmental monitoring program with a baseline/target will be more specific as well as more effective in assessing the overall sanitary conditions of the facility.
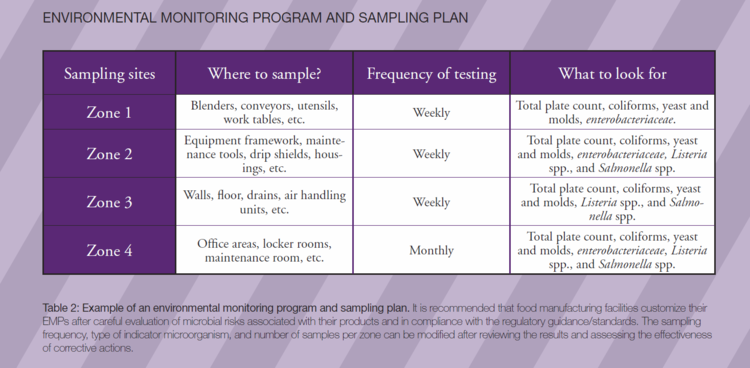
It is critical for the EMP team to define what constitutes highest risk areas (zone 1) to lowest risk areas (zone 4) for product contamination in a facility. Also, it is important to choose the right testing tools and methods before beginning to collect samples.
It also is critical for food manufacturers to develop a science-based environmental testing and verification program that effectively monitors the overall hygiene quality of the facility. Table 1 provides a general overview of environmental zones in a food manufacturing plant. In general, most environmental swab samples should be taken from zones 2 to 3, and fewer samples should be taken from zone 4.However, the frequency and number of samples per zone should be modified after reviewing the results and effectiveness of corrective actions.

Indicator Microorganisms
Indicators are non-pathogenic microorganisms that may be naturally present in food or the environment. These indicator organisms are used to assess the overall sanitation or environmental condition that may signal or indicate the potential presence of pathogens that may cause significant health risks to consumers. Zone 1 is tested for indicator organisms, and zones 2 to 4 are tested for indicators and pathogens. The following reasons explain some of the advantages of using indicator organisms in an EMP:
- They are less expensive and save time compared to pathogens.
- Low prevalence of pathogenic microorganisms limits the practical significance of direct pathogen testing.
- Indicator microorganisms are high in numbers and can be easily enumerated.
- Indicator microorganisms are a valid representative of pathogens of concern since indicators use nearly the same pH, nutrients, temperature, water, etc. as that of pathogens.
- They are non-pathogenic, so there is not a need for sophisticated containment facilities/labs (e.g., Bio Safety Level-2) for sample analysis.
Indicator organisms are not a substitute for testing for pathogens. A positive result indicates possible contamination and a risk of foodborne disease. Examples of some of the indicator microorganisms that can be used to monitor hygienic conditions are total aerobic plate count, total coliforms, fecal coliforms, and Enterococcus spp. of fecal origin. Table 2 provides a general overview of EMP in a food manufacturing facility with details of probable indicator microorganims and sampling frequencies.
Establishing a Baseline and Corrective Actions
Historical data, such as the previous six to 12 months of consecutive data, is needed to establish a baseline/target. For example, if a site tests <50 cfu for a year with two or three spike readings, then 50 cfu would be set as the baseline.
The environmental monitoring program and the target/baseline are unique for each plant and for each type of product. Also, it is different for different zones (1 to 4). Since the reasons for a positive finding are likely to be plant specific, the corrective action will differ from plant to plant based on final food products.
It is important to compare the environmental sampling results against a target level or a baseline. Any increase in microorganisms or pathogen number should be monitored. These results signal a possible deviation in the sanitary conditions. An appropriate corrective action (e.g., change of sanitizers, change in sanitizing frequencies, etc.) should be initiated to bring the values close to or below the target/baseline. Further, it requires monitoring to check the indicator or pathogenic microorganism level in the plant.
Typical Corrective Actions for Pathogen Positives
Scenario I: Positive zone 1
- Stop production in the affected line.
- Stop product.
- Thoroughly examine the area, both visually and through vector swabbing.
- Breakdown production lines for inspection and take appropriate corrective actions (e.g., leakage, employee traffic, etc.).
- Thoroughly clean affected site (50-foot radius) and swab site and adjacent areas (zones 2 and 3).
- Increase sampling frequency (e.g., from weekly to daily) until you get three consecutive negative results.
Zone 1 is normally tested for indicator microorganisms. Testing zone 1 for pathogens such as Salmonella is normally done only for special situations. If zone 1 tests positive for a pathogen, then the product made on that line must be held until further test results that confirm the pathogen are available, and it will most likely initiate a recall situation. Therefore, it is important to have a predetermined action plan that would be implemented in case of a Salmonella (or any pathogen) positive zone 1. The response team and appropriate management should make a careful decision on disposition of finished product, which is put on hold as a result of zone 1 positive. If possible, the product should be reworked or condemned according to all legal and regulatory procedures.
Scenario II: Positive zone 2
- Breakdown production lines for inspection.
- Restrict traffic flow in these areas to the extent possible.
- Thoroughly examine the area, both visually and through vector swabbing.
- Take appropriate corrective actions (e.g., leakage, employee traffic, etc.).
- Collect swabs after thorough cleaning (zones 2 and 3) in a 50-foot radius
- Increase sampling frequency (e.g., from weekly to daily) until you get three consecutive negative results.
- Zone 1 swabbing and/or finished product testing may need to be initiated or intensified in the event of persistent zone 2 positives.
Scenario III: Positive zone 3 and negative zone 2
This is an early indication that the cleaning and sanitation programs need to be more robust or redesigned.
- Restrict traffic flow in these areas to the extent possible.
- Thoroughly examine the area.
- Collect swabs after thorough cleaning (zone 3) in a 50-foot radius.
- Increase sampling frequency (e.g., from weekly to daily) until you get three consecutive negative results.
Scenario IV: Positive zones 2 and 3
- Break down production lines for inspection.
- Restrict traffic flow in these areas to the extent possible.
- Thoroughly examine the area both visually and through vector swabbing.
- Take appropriate corrective actions (e.g., leakage, employee traffic, etc.).
- Collect swabs after thorough cleaning (zones 2 and 3) in a 50-foot radius.
- Increase sampling frequency (e.g., from weekly to daily) until you get three consecutive negative results in zones 2 and 3.
- Zone 1 swabbing and/or finished product testing may need to be initiated or intensified in the event of persistent zone 2 positives.
Positive Results - Action Plan Procedures
- Reassemble your team.
- Initiate root-cause investigation. (What happened?)
- Use the team’s findings to improve operations, such as:
- Thoroughly examine the area, both visually and through vector swabbing.
- Increase cleaning and sanitation frequencies or modify method.
- Reexamine employee traffic patterns and redirect, if feasible.
- Make repairs (e.g., leaks, cracks etc.).
- Audit production handling practices (e.g., product and material handling, etc.).
- Redesign and/or perform equipment maintenance as needed to eliminate harborage niches that may act as a source of contamination or facilitate cleaning access.
- Conduct indirect cleaning such as floor scrubbing and sanitation or cleaning of overhead equipment, A/C ducts and pipes, etc.
- Increase the swab frequencies.
- Verify the effectiveness.
- Monitor and document.
In a rare and extreme situation, where a “hot zone” cannot be eliminated, the area should be physically quarantined from the rest of the plant until a permanent solution can be found.
Mapping
A map of all sampling locations on a facility design diagram is an effective way of identifying hot spots to take appropriate corrective actions. Map the locations of any negative (green color flag), increasing trend (yellow color flag), and positive samples/results (red color flag) on a facility design diagram to help define the scope of the problem. Mapping helps in identifying harborage niches and hot spots that may act as a source of contamination.
Summary

In recent years, it has become increasingly critical for food industries to implement effective hazard control programs as both USDA and FDA have become far more aggressive in implementing risk-based preventive control procedures even before FSMA is fully implemented.
A properly established environmental monitoring program will act as an early warning system for potential microbial hazards in a food manufacturing plant and confirm that sanitary designs, personnel practices, and operational methods already in place are, in fact, effective.
Apart from the food-contact surfaces and nonfood-contact surfaces discussed above, the air, water, and even plant employees also can act as potential sources of contamination. Periodic air, water, and plant employee hand swab samples should be monitored for indicators as well as pathogens.
If you find a high level of microorganisms, then appropriate corrective actions (including employee hygienic training) should be implemented to avoid product contamination.
Although FSMA’s proposed preventive controls rule did not include requirements for environmental monitoring or finished product testing, it is understood that the food manufacturer needs to develop and implement an effective EMP that is capable of detecting signs of microbial contaminants as early as possible and initiate appropriate corrective actions, thus eliminating or reducing the potential microbial hazard and assuring safety of the product.

