Differences of HACCP and HARPC
The clock is ticking for compliance with FSMA’s Hazard Analysis and Risk Based Preventive Controls (HARPC) requirements. There are exceptions for small businesses, but the compliance deadline for most companies was September 17, 2016. This includes successfully completing documented hazard analysis and implementing preventive controls (PCs) for known or foreseeable hazards associated with recalls for your product type as cited in the FDA’s Annual Reportable Food Registry.
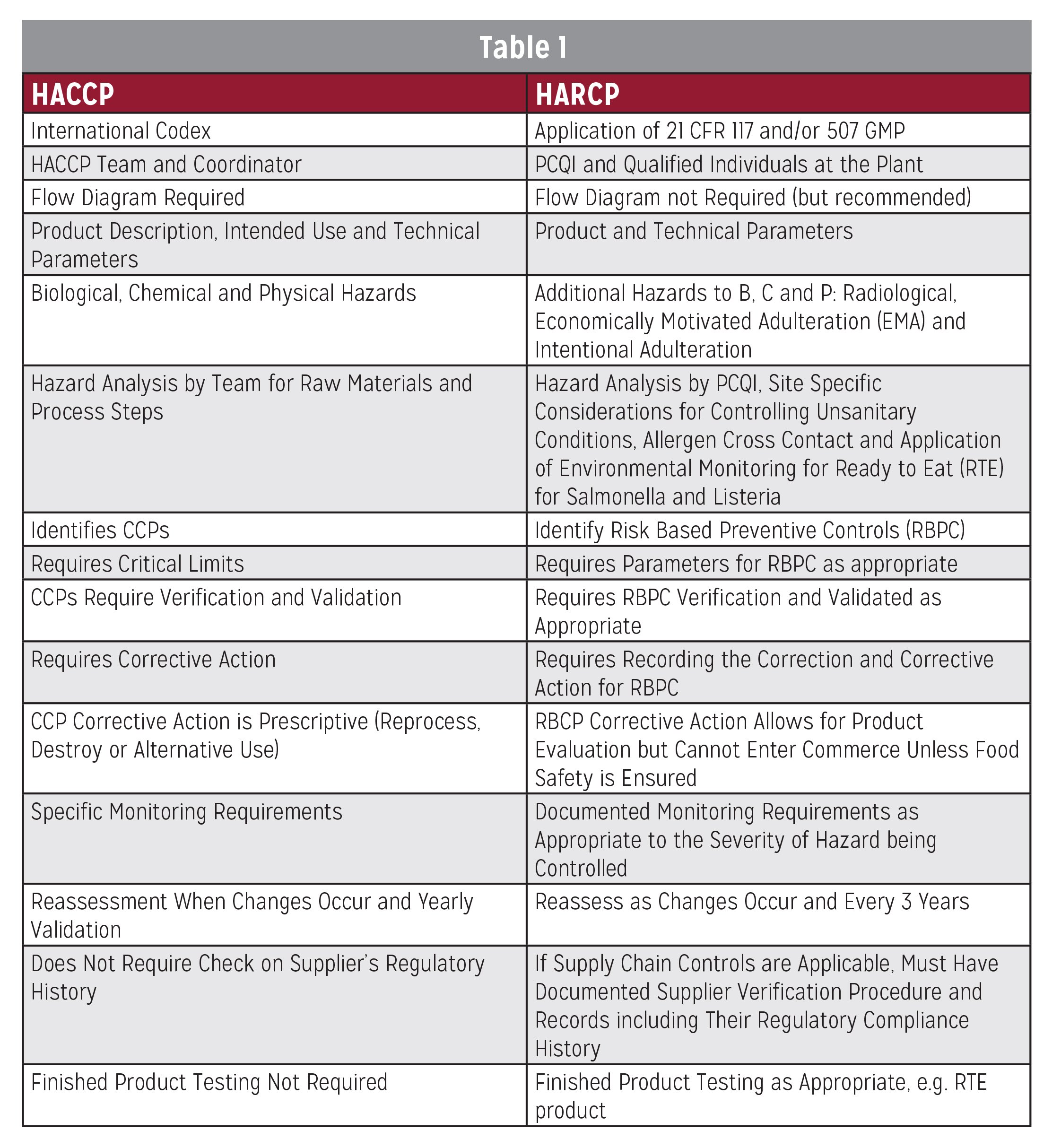
What are you planning to show the state or FDA inspectors who will ask to see your food safety plan? If you are planning to show them your HACCP plan you may want to reevaluate that strategy. Although HACCP and HARPC are based on similar concepts, they are not the same.
Your hazard analysis and preventive control implementation must be completed or overseen by your Preventive Control Qualified Individual (PCQI). Your PCQI could attend an FDA-recognized curriculum or be otherwise qualified by education, background and/or experience. Your hazard analysis must be documented regardless of the outcome; even if you didn’t identify any additional preventive controls that need to be monitored similar to a CCP.
Table 1 compares HACCP and HARPC. Additional details about HARPC hazard analysis will be necessary to understand the regulation’s intent to prevent food safety recalls.
The most important aspect of HARPC is the hazard analysis requirements for documenting risk-based preventive controls (RCPC) and if the supply chain control program is applicable for the raw materials and products shipped. You must conduct hazard analysis to identify and evaluate known or foreseeable hazards for each type of food manufactured, processed, packed or held at your facility to determine hazards that require a preventive control. Your hazard analysis will be based on experience (site specific), illness data, scientific reports and guidance documents from industry, regulators, universities, etc. The hazard analysis must consider the likelihood (probability) and severity that these hazards could or would occur in the absence of a preventive control. The hazard evaluation must consider the effect of the following on the safety of the finished food for the intended customer:
- Formulation of the food
- Condition, function and design of the facility and equipment (site evaluation)
- Raw materials and other ingredients
- Transportation practices
- Manufacturing/processing procedures
- Packaging and labeling activities
- Storage and distribution
- Intended or foreseeable use
- Sanitation, including employee hygiene
- Any other relevant factors, seasonal or weather variation affecting conditions that allow for or increase the potential for hazards, e.g. levels of a natural toxin
This evaluation will need to be documented, particularly the site evaluation, to identify any site-specific PCs that must be applied to produce safe food. Modified requirements contained in Subpart D pertain to the storage of fully packaged refrigerated products that need temperature to control pathogens and the expectation for documented temperature control records.
If your company is relying on a supplier or customer to control an identified hazard, then the supply chain program will be applicable. If your supplier is controlling the hazard you will have a supplier verification program in place to review the adequacy of their controls including their regulatory history. If you are relying on your customer to control the identified hazard you will have documented assurances and description of those controls that must be updated on an annual basis. The FDA has noted that the supply chain program can be complicated and has issued enforcement discretion guidance in addition to the draft guidance for implementation of said requirements. For a full explanation please visit https://www.fda.gov/food/guidanceregulation/fsma/ucm253380.htm.
Regulators have the right to review your HARPC hazard analysis, food safety procedures, training and records for your preventive controls. It is always in your best interest to have food safety records that accurately reflect the sanitary conditions at your plant and have documented evidence for improvements made while implementing your food safety plan(s). In addition, it is advised that you test your readiness for a FSMA regulatory inspection either internally or by using a third-party consultation to verify the ability to retrieve/present food safety records, the accuracy/omissions in those record, and the ability of personnel to answer interview questions about your company’s food safety plan.